
31-Oct-2025
Presto Instruments
A spectrophotometer is an analytical instrument used to take measurements of the amount of light that is absorbed, as well as transmitted by a sample at a given wavelength. It can then be used in the form of spectrophotometry to measure a liquid and help determine the concentration, purity, or chemical structure of a sample.
By measuring how a sample interacts with light, any number of aspects of a compound can be identified and measured, concentration and absorbance being the most common, making it very useful for the biological, chemical, and environmental sciences.
The spectrophotometer works on the principle of Beer-Lambert Law, which states that the amount of light collected by a substance is directly proportional to the concentration and the distance the light path travels through. The spectrophotometer does this by producing a monochromatic light source on a provided sample and measuring the intensity of light that passes.
The absorbance reading of the sample is the transmitted light intensity in addition to the incident light intensity. The components of the spectrophotometer can be described as: a light source, a light selection mechanism (monochromator), a cuvette (sample holder), and a detector measuring absorbance or transmittance.
The principle of the spectrophotometer works on the Beer-Lambert Law, which is denoted by A = 𝜖𝑐𝑙. This is the law that defines that the absorbance (A) of the solution will be proportional to its concentration (c) and the path length the light has while passing through it (l). The equation also consists of 𝜖, the molar absorptivity, which is constant for a particular substance at a certain wavelength.
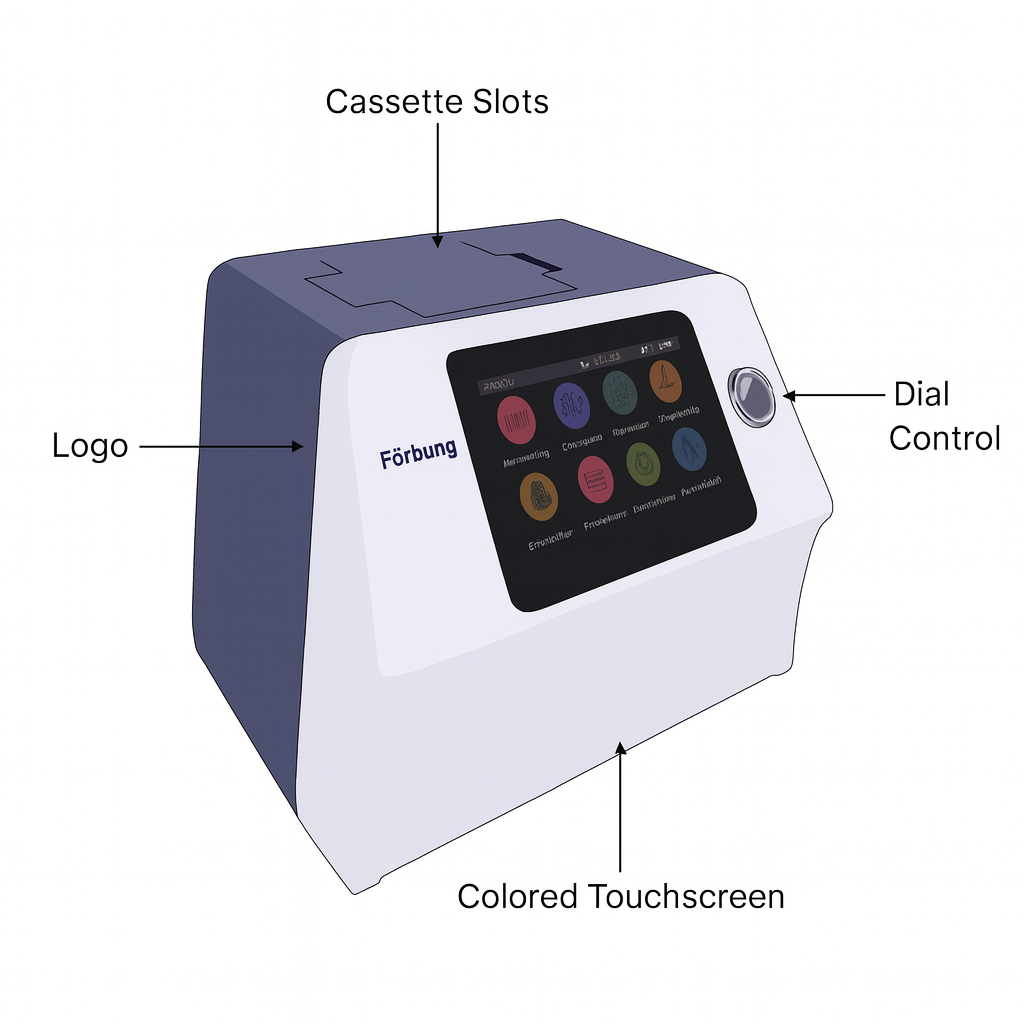
A spectrophotometer has a light source for supplying light, a monochromator for selecting a particular wavelength, a sample holder for the cuvette with the sample, a detector to measure the transmitted light, and a display to indicate the measurement.
Important parts of a Spectrophotometer:-
|
Component |
Function |
|
Light Source |
Produces a wide range of light, from a tungsten lamp to produce visible light or a deuterium lamp to produce UV light. |
|
Monochromator |
Breaks the light down into its different wavelengths. It typically uses a prism or a diffraction grating to select one particular, individual wavelength to transfer through the sample. |
|
Slit |
This process focuses the light beam into a highly specific wavelength, thereby improving the precision of the measurement. |
|
Sample Holder (cuvette) |
A minimal, clear tube in which the liquid sample is stored. The light beam travels through the cuvette and the sample. |
|
Detector |
It keeps track of the quantity of light that has penetrated the sample. It changes the light into an electric current. |
|
Display/Processor |
It transforms the electric current signal from the detector into a readable measurement, such as absorbance or transmittance, and then presents it on a digital display. |
There are several types of spectrophotometers, including UV-Visible (UV-Vis), Infrared (IR), Fluorescence, and Near Infrared (NIR). These different types of spectrophotometers will be described here in detail.
A UV-Visible spectrophotometer is an instrument that measures the amount of Ultraviolet (UV) and visible light that is absorbed or transmitted by a sample. It is standard analytical equipment to calculate the concentration of the substance and give information on its composition and optical properties. The collected data is referred to as a UV-Vis spectrum, which is akin to a "fingerprint" for the substance.
An infrared (IR) spectrophotometer is a measuring device that uses infrared light to define chemical bonds and functional groups in a sample. The infrared spectrophotometer detects the energy absorbed by a group of infrared photons passing through a sample and makes a unique "fingerprint" spectrum connected with the vibrational states of the molecule. The infrared technique is commonly used to identify unknown material and to give structural identity to a compound.
A fluorescence spectrophotometer is a measuring device that operates by measuring light emitted from the sample after it is charged with a specific wavelength of light. In the measurement, the activation light from a light source provides energy to the sample, and the light emitted from the sample (fluorescent light) passes through an emission monochromator to separate the emitted light, after which the light is calculated by detection.
These devices are used in a variety of fields to analyze materials, understand properties of fluorescent molecules and conduct research in biochemistry, environmental science, and materials science.
A Near Infrared (NIR) Spectrophotometer is an analytical instrument that uses near-infrared light to assess the chemical composition of a sample and performs analysis without destruction. This method uses the absorption of NIR light (most often 700–2500 nm) to assess the presence and amount of specific functional groups, such as C-H, N-H, and O-H. This general method is widely used in agriculture, food and pharmaceutical industries to quickly assess quality control.
The central process contains five main elements operating in succession:
Spectrophotometers are helpful in different industries, such as the pharmaceutical industry, food and beverage industries, the chemical industry, environmental testing, textile industries, and the paint and coatings industries. Here is the detailed description:
During formulation, drug concentration, purity, and stability are measured utilizing spectrophotometers. Spectrophotometers can supplement quality by determining drug dosing and drug heterogeneity through the measurement of active pharmaceutical ingredients and excipients at a given UV-Vis absorbance.
Spectrophotometers measure foods for color, additives, and nutritional composition–including the measurements of proteins, sugar, and vitamins. They measure quality for contamination, and when foods are to undergo manufacturing and packaging processes, they maintain stability in color and flavor properties of the food.
Spectrophotometers assess samples of air, soil, and water to determine contaminants, including nitrates, phosphates, and heavy metals. These instruments are invaluable for environmental monitoring to ensure compliance with safety regulations and in cases of pollution control programs docking screening.
Spectrophotometers can determine and match the colors of textiles. They provide guarantees that colors within dye lots are consistent, signal differences in shades, and provide for color uniformity in textiles particularly important in adherence to brand color standards.
Spectrophotometers serve as a practical application for color, transparency, and reflectance measurement, allowing paint and coating producers to ensure color consistency. They ensure that a finished product meets customer standards and visual expectations.
Spectrophotometers are valuable instruments across a wide range of industries, including science, because they provide accurate quantities for not only material evaluation but also for determining their amounts. In general, spectrophotometers are laboratory instruments that are safe, accurate, and easy to operate for research and quality analysis, as well as in environmental studies. Therefore, knowing how the instrument works, the principles of the instrument, and the parts of the spectrophotometer will give you the ability to carry out a laboratory/field analysis accurately and easily.
Elevate your quality assurance process to new heights
At Presto, we take pride in being Global manufacturers of Laboratory Testing Instruments for different industries.